NovoBiotic Technology for Previously “Unculturable” Organisms
Our in situ cultivation technology allows us to access novel microorganisms from soil and marine environments (Kaeberlein et al., Science 2002). These microorganisms are grown in a special diffusion chamber placed back in their natural environment, where essential growth factors can diffuse into the chamber. By repeatedly transferring cultures from one chamber to another, we generate “domesticated” variants that can thrive under standard laboratory conditions. Using this method, we have cultivated thousands of novel microorganisms, including many new species and genera that are only distantly related to known microorganisms. Multiple drug leads have already been identified from this unique collection.
Since developing the original diffusion chamber, we have advanced our methods with new culturing technologies that expand access to unique microbes. These technologies include the “iChip”, a miniaturized chamber that enables the isolation and cultivation of new microbes in a single step. A modified version of the diffusion chamber, the “trap”, selectively captures filamentous microbes, the most prolific producers of secondary metabolites. Using all our unique culturing technologies, we have built a strain collection of over 75,000 microbial isolates.
Objective research is of paramount importance to NovoBiotic Pharmaceuticals to ensure public trust and meet scientific, programmatic and ethical goals of our National Institutes Health (NIH) grant efforts. To view NovoBiotic Pharmaceuticals Financial Conflict of Interest Policy, click here.
In accordance with the latest NIH guidelines, minutes documenting discussions and decisions related to the company’s biosafety and biosecurity oversight by NovoBiotic’s Institutional Biosafety Committee (IBC) are available here.
Compounds
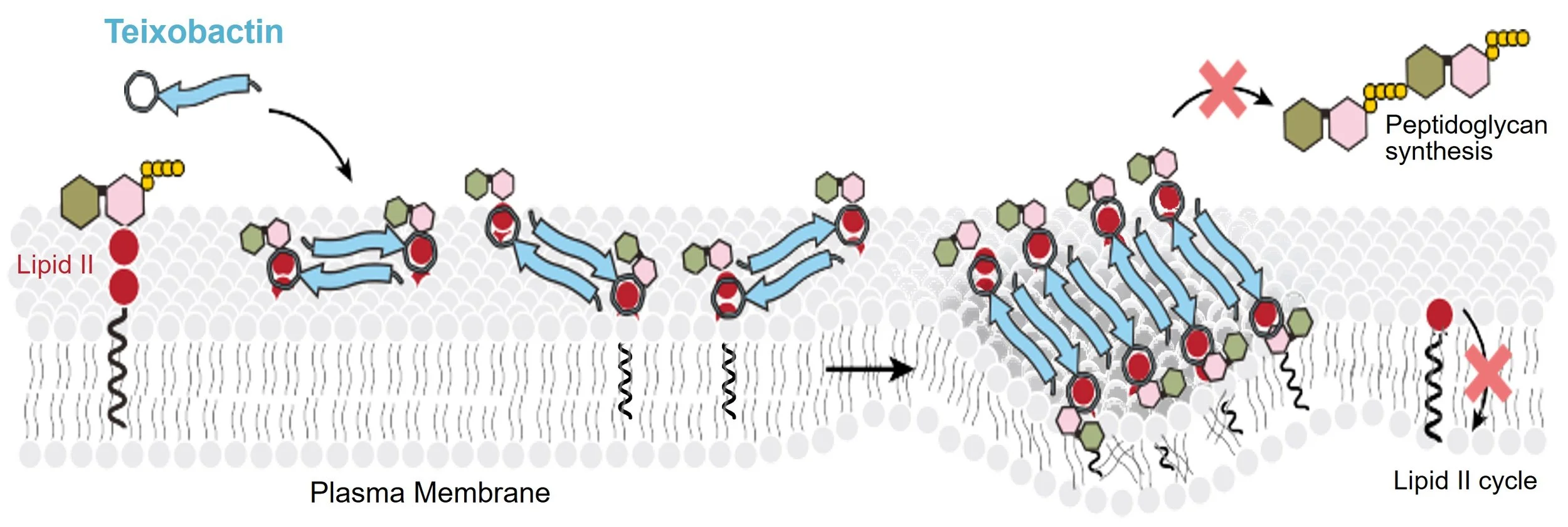
Teixobactin. Teixobactin is the first member of a new class of antibiotic that was discovered by NovoBiotic using the iChip technology (Ling et al., 2015). The compound is highly potent against a broad range of Gram-positive microbes, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Bacillus anthracis. Teixobactin shows excellent activity in several animal models of infection. When delivered by inhalation, teixobactin was extremely effective against nontuberculous mycobacteria (NTM) lung infections in animal models. Lung NTM infections can be devastating to patients with structural lung diseases such as cystic fibrosis, and there are very few effective drugs available.
Teixobactin is a bacterial cell wall synthesis inhibitor that acts by binding two different targets - lipid II (peptidoglycan precursor) and lipid III (teichoic acid precursor). It binds to the undecaprenyl-PP-sugar region of these precursors, which is not known to be subject to mutation. In addition, teixobactin binds to the bacterial cell membrane, and self-aggregates into massive, irreversible macromolecular structures that weaken the membrane, which contributes to its potent cell killing ability (Shukla, 2022). As a result, teixobactin is the first example of a target-specific compound essentially free of resistance. Teixobactin is in late preclinical development.
Source: Shukla, R., Lavore, F., Maity, S. et al. Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature 608, 390–396 (2022).
Clovibactin. Clovibactin is another new antimicrobial compound discovered using NovoBiotic’s unique culturing platform. It is in preclinical development for treating a wide range of bacterial infections caused by Gram-positive pathogens including Staphylococcus aureus, Streptococcus pneumoniae, and Bacillus anthracis. Like teixobactin, Clovibactin rapidly kills bacteria by inhibiting bacterial cell wall synthesis. No spontaneous resistance has been detected and serial passaging studies in sublethal concentrations of clovibactin failed to generate resistance. Although similar in structure to teixobactin, clovibactin is smaller and shows different binding characteristics to cell wall precursors. Clovibactin demonstrated excellent efficacy against MRSA in two mouse models of infection (septicemia and thigh infection), and low toxicity. All these results demonstrate promising potential for this exciting new compound to treat drug-resistant infections.
Publications
Malkawi E, Parmar A, Das S, Newire E, Jones CM, Morrison KA, Karak M, Blanc F, Harper N, Lakshminarayanan R, Poh ZS, Verma NK, Unsworth J, Hughes DE, Ling LL, Cochrane SA, Hope W, Singh I. Novltex: A New Class of Antibiotics with Potent Activity against Multidrug-Resistant Bacterial Pathogens─Design, Synthesis, and Biological Evaluation. J Med Chem. 2025 Sep 16. doi: 10.1021/acs.jmedchem.5c01193. Epub ahead of print. PMID: 40957081. [PDF]
Lawrence WS, Peel JE, de Winter R, Ling LL, Nitti AG, Peoples AJ, Shukla R, MacGillavry HD, Heine HS, Hensel ME, Whorton EB, Weingarth M, Lewis K, Hughes DE. Teixobactin: A Resistance-Evading Antibiotic for Treating Anthrax. ACS Infect Dis. 2025 Feb 27. doi: 10.1021/acsinfecdis.4c00835. Epub ahead of print. PMID: 40014033. [PDF]
Shukla R, Lavore F, Maity S, Derks MGN, Jones CR, Vermeulen BJA, Melcrová A, Morris MA, Becker LM, Wang X, Kumar R, Medeiros-Silva J, van Beekveld RAM, Bonvin AMJJ, Lorent JH, Lelli M, Nowick JS, MacGillavry HD, Peoples AJ, Spoering AL, Ling LL, Hughes DE, Roos WH, Breukink E, Lewis K, Weingarth M. Teixobactin kills bacteria by a two-pronged attack on the cell envelope. Nature. 2022 Aug;608(7922):390-396. doi: 10.1038/s41586-022-05019-y. Epub 2022 Aug 3. PMID: 35922513; PMCID: PMC9365693. [PDF]
Krumberger M, Li X, Kreutzer AG, Peoples AJ, Nitti AG, Cunningham AM, Jones CR, Achorn C, Ling LL, Hughes DE, Nowick JS. Synthesis and Stereochemical Determination of the Peptide Antibiotic Novo29. J Org Chem. 2023 Feb 17;88(4):2214-2220. doi: 10.1021/acs.joc.2c02648. Epub 2023 Jan 19. PMID: 36655882; PMCID: PMC9942206. [PDF]
Wirtz DA, Ludwig KC, Arts M, Marx CE, Krannich S, Barac P, Kehraus S, Josten M, Henrichfreise B, Müller A, König GM, Peoples AJ, Nitti A, Spoering AL, Ling LL, Lewis K, Crüsemann M, Schneider T. Biosynthesis and Mechanism of Action of the Cell Wall Targeting Antibiotic Hypeptin. Angew Chem Int Ed Engl. 2021 Jun 7;60(24):13579-13586. doi: 10.1002/anie.202102224. Epub 2021 May 7. PubMed PMID: 33768646; PubMed Central PMCID: PMC8252469. [PDF]
Espinoza JL, Dupont CL, O'Rourke A, Beyhan S, Morales P, Spoering AL, Meyer KJ, Chan AP, Choi Y, Nierman WC, Lewis K, Nelson KE (2021) Predicting antimicrobial mechanism-of-action from transcriptomes: A generalizable explainable artificial intelligence approach. PLoS Comput Biol. 17(3). PMID: 33780444 [PDF]
Wirtz DA, Ludwig KC, Arts M, Marx CE, Krannich S, Barac P, Kehraus S, Josten M, Henrichfreise B, Müller A, König GM, Peoples AJ, Nitti A, Spoering AL, Ling LL, Lewis K, Crüsemann M, Schneider T (2021) Biosynthesis and Mechanism of Action of the Cell Wall Targeting Antibiotic Hypeptin. Angew Chem Int Ed Engl. PMID: 33768646 [PDF]
Quigley J, Peoples A, Sarybaeva A, Hughes D, Ghiglieri M, Achorn C, Desrosiers A, Felix C, Liang L, Malveira S, Millett W, Nitti A, Tran B, Zullo A, Anklin C, Spoering A, Ling LL, Lewis K (2020) Novel Antimicrobials from Uncultured Bacteria Acting against Mycobacterium tuberculosis. mBio. Aug 4 11(4):e01516-20. PMID: 32753498 [PDF]
Lawrence WS, Peel JE, Sivasubramani SK, Baze WB, Whorton EB, Beasley DWC, Comer JE, Hughes DE, Ling LL, Peterson JW (2020) Teixobactin Provides Protection against Inhalation Anthrax in the Rabbit Model. Pathogens. Sep 22 9(9):773. PMID: 32971758 [PDF]
O'Rourke A, Beyhan S, Choi Y, Morales P, Chan AP, Espinoza JL, Dupont CL, Meyer KJ, Spoering A, Lewis K, Nierman WC, Nelson KE (2020) Mechanism-of-Action Classification of Antibiotics by Global Transcriptome Profiling. Antimicrob Agents Chemother. Feb 21 64(3):e01207-19. PMID: 31907190 [PDF]
Öster C, Walkowiak GP, Hughes DE, Spoering AL, Peoples AJ, Catherwood AC, Tod JA, Lloyd AJ, Herrmann T, Lewis K, Dowson CG, Lewandowski JR (2018) Structural studies suggest aggregation as one of the modes of action for teixobactin. Chem Sci. Sep 20 9(47):8850-8859. PMID: 30627403 [PDF]
Jones MB, Nierman WC, Shan Y, Frank BC, Spoering A, Ling L, Peoples A, Zullo A, Lewis K, Nelson KE (2017) Reducing the bottleneck in discovery of novel antibiotics. Microbial Ecology. 73(3):658-667. PMID: 27896376. [PDF]
Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A , Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR , Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K (2015) A novel antibiotic kills pathogens without detectable resistance, Nature, 07 January 2015, doi:10.1038/nature14098. [PDF]
Gavrish E, Sit CS, Cao S, Kandror O, Spoering A, Peoples A, Ling L, Fetterman A, Hughes D, Bissell A, Torrey H, Akopian T, Mueller A, Epstein S, Goldberg A, Clardy J, Lewis K (2014) Lassomycin, a ribosomally synthesized cyclic peptide, kills Mycobacterium tuberculosis by targeting the ATP-dependent protease ClpC1P1P2. Chem Biol. 21(4):509-18. [PDF]
Buerger S, Spoering AL, Gavrish E, Leslin C, Ling LL, and Epstein S. (2012) Microbial scout hypothesis and microbial discovery. AEM. 78(9):3229-33. [PDF]
Buerger S, Gavrish E, Spoering AL, Leslin C, Ling L,L. and Epstein S. (2012) Microbial scout hypothesis, stochastic exit from dormancy, and the nature of slow growers. AEM. 78(9): 3221-8. [PDF]
Lewis, K, Epstein, S, D’Onofrio, A, and Ling, LL (2010) Uncultured microorganisms as a source of secondary metabolites. J. Antibiot (Tokyo) 63(8):468-76.
Zhang, Q, Peoples, AJ, Rothfeder, M T, Millett ,WP, Pescatore BC, Ling LL, and Moore CM (2009) Isofuranonaphthoquinone produced by an actinoplanes isolate. J. Nat. Prod. 72(6):1213-5. [PDF]
Peoples, AJ, Zhang, Q, Millett, WP, Rothfeder, MT, Pescatore, BC, Madden, AA, Ling LL, and Moore CM (2008) Neocitreamicins I and II, novel antibiotics with activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci. J. Antibiot (Tokyo) 61(7):457-63. [PDF]
Kaeberlein, T, Lewis, K, and Epstein, SS (2002) Isolating "Uncultivable" Microorganisms in Pure Culture in a Simulated Natural Environment. Science 296:1127-1129. [PDF]